Hereditary Hemochromatosis(HH), is an autosomal recessive genetic disorder that places the affected individual at increased risk of iron overload. There are several variations of HH, with classical or type 1 Hereditary Hemochromatosis related to mutations C282Y & H63D on the HFE gene, being the most common(Flemming et.al. 2005). Classical Hereditary Hemochromatosis will be the focus of this paper, and will be referred to as HH unless otherwise noted. HH and elevated iron stores have been linked with several diseases or conditions including; cirrhosis of the liver, arthritis, diabetes, depression, fatigue and other hormonal issues(Pilling, L. C. et al. 2019). With proper management these symptoms can be ameliorated.
i. Human disease or illness
Carriers of HH HFE gene mutations C282Y or H63D are at increased risk of iron overload, or a toxic build up of unbound iron. Chronic, untreated iron overload can place individuals at increased risk of; chronic liver disease or cirrhosis, heart failure, heart arthymaias, hypogoanadism, diabetes, arthritis, fatigue, depression and hypermelanotic pigmentation of the skin (Pilling, L. C. et. al. 2019)(Adams, P. C., Deugnier, Y., Moirand, R., & Brissot, P. 1997). Additionally, carriers may be at increased risk of infection of specific bacteria. Left untreated iron overload leads to the progressive accumulation of iron in the parenchymal cells or the liver, heart, and heart and eventual organ failure(Bacon, Powell, Adams, Kresina, & Hoofnagle 1999).
Iron is a transition metal, with the atomic symbol Fe, and is vitally important for cellular respiration(Dixon, S. J., & Stockwell, B. R. 2013). However, free or unbound iron has also been found to promote the formation of reactive oxygen species(ROS)(Dixon, S. J., & Stockwell, B. R. 2013). ROS along with iron are mediators of cell death, and contribute to enhanced oxidative stress to surrounding tissues and organs(Dixon, S. J., & Stockwell, B. R. 2013). Unbound iron, or iron not bound to transferratin, or heme proteins causes damage to DNA, proteins, or lipids(Dixon, S. J., & Stockwell, B. R. 2013).Through this enhanced oxidative stress, and cell death its believed HH and its elevated iron stores cause the associated organ damage.
Prevalence and Penetrance
C282Y & H63D HFE gene mutations are primarily found in white or caucsian individuals specifically of northern European decent(Pilling, L. C. et. al. 2019). HFE related HH is the most commonly found genetic inheritance disease(Rs1799945). Both C282Y & H63D HFE gene mutations being autosomal recessive there is significant variability between hetero- and homozygous genotypes and the development of HH(Pilling, L. C. et. al. 2019). Additionally there is significant differences how common each mutation is, their penetrance, and degree of sexual dimorphisim(Pilling, L. C. et. al. 2019).
C282Y mutations make up 95% of HH cases diagnosed, appearing in 10-15% of individuals of northern European decent(Pilling, L. C. et. al. 2019)(Flemming RE et al. 2005). Of northern Europeans 1/150 have have been found to be homozygous genotypes for the C282Y gene(Pilling, L. C. et al. 2019). H63D HFE mutations account for only 4% of all diagnosed cases of HH, and only result in a less severe form of HH(Pilling, L. C. et. al. 2019).
Although the HFE gene is not sex linked, there is significant disparity between males and females and the development of HFE linked HH. This disparity may be due to estrogen effects on iron absorption, in addition to menstrual losses(Penny A. Dacks 2012), not in the prevalence of C282Y or H63D gene mutation. Of men that were C282Y homozygous, 28.4 – 38.3% have been found to develop HH(Allen KJ et al. 2008)(Pilling, L. C. et al. 2019). HH is more likely to be diagnosed later in life as health concerns from iron overload develop only after a significant lag time. C282Y homozygous men and women between the ages of 40 to 70 had the highest prevalence of HH with an odds ratio of 411.1(Pilling, L. C. et al. 2019).This finding further supports estrogens protective effects in relation to iron absorption. C282Y homozygous women only 1.2% met diagnoses criteria for HH(Allen KJ et al. 2008). No differences in in the development of diabetes, heart, or liver diseases have been found between men and women with diagnosed HH(Pilling, L. C. et al. 2019).
H63D mutations are more common than C282Y mutation with 15-40% of the Caucasian population possessing this allele(Flemming RE et al. 2005). However H63D HFE linked mutations result in a significantly less severe form of HH than C282Y HFE linked mutations, and account for only a small portion of HH diagnoses(Flemming RE et al. 2005). H63D mutations often lead to higher than average iron saturation, but not regularly to clinically significant levels(Flemming RE et al. 2005).
Philling, L. C. et al. (2019) found no association between neither C282Y nor H63D heterozygous genotypes in studied male patients and diagnoses of HH, given the remaining allele was not predictive of HH. However there was a slight association found by Philling L. C. in female C282Y heterzygotes(2019). Mortality was not increased in C282Y heterozygotes compared with participants with no C282Y mutations.
While C282Y and H63D heterozygous individuals are not at an increased risk of HH, these mutations are not mutually exclusive and its possible to have both. Compound C282Y/H63D heterzygotes are at an increased risk of developing HH as compared to the general population(Flemming RE et al. 2005). It is estimated that C282Y/H63D compound heterozygotes have a 200% lower risk of HH than their homozygous C282Y counterparts(Flemming RE et al. 2005). This risk is with the assumption that these mutations are found on separate alleles.
Diagnosis
Diagnoses of HH relies on screening of at risk populations, and those that present with any of the commonly associated illness such as liver failure, diabetes. Screening can be preformed using DNA sequencing and pedigrees(Bryant, et al. 2009), while diagnoses can be made using blood tests, MRI scans of the liver, or liver biopsies(Nichols & Bacon 1989).
Blood tests are often the first step in the diagnosis of HH, in individuals exhibiting symptoms, or at increased risk due to family history(Hemochromatosis 2018). Total iron binding capacity(TIBC), serum iron, and ferritin levels are the most useful blood measurements(Nichols & Bacon 1989). TIBC, is a measure of the amount of iron capable to be bound to the transferritin protein, a protein that carries iron in the blood(TIBC, UIBC, and Transferrin). Normal TIBC values are 250 – 370 μg/dL, lower values are indictive of increased risk of HH. Serum iron measures the total amount of iron bound in the blood(TIBC, UIBC, and Transferrin). Normal values for serum iron are 65–177 μg/dL for males and 50–170 μg/dL for females. Serum ferritin is a measure of the ferritin protein in the blood, which is a reflection of total body iron(TIBC, UIBC, and Transferrin) or liver stores(Hemochromatosis 2018). Normal levels for serum ferritin are 20-250 μg/L for males, and 15-150 μg/L for females. Additionally serum iron can be divided by TIBC, and can be expressed as a percentage and called transferritin saturation(TIBC, UIBC, and Transferrin). Values higher than 45% are considered indicative of HH(Hemochromatosis 2018).
Liver biopsy, or MRI of the liver can also be used to determine the liver damage, and the degree of iron saturation(Hemochromatosis 2018). MRI scans are a non-invasive measure which can provide some insight to iron overload, but are often cost prohibitive. Liver biopsies, are an accurate way of determining iron stores, they are invasive and come with increased risks compared to other measures(Hemochromatosis 2018).
DNA screening, like pedigrees, is not effective in diagnosing HH in individuals, but may be a cost effective means of determining who is at risk(Bryant, et al. 2009). C282Y homozygotes have the highest likelihood of receiving a HH diagnoses. However only 28.4 – 38.3% have been found to develop HH(Allen KJ et al. 2008). DNA screening, and pedigrees may allow for early intervention in at risk individuals limiting possibly permanent organ damage from iron overload.
Treatment
Treatment of HH is centered around early detection before any significant organ damage has already occurred. Once HH has been diagnosed, iron stores are attempted to be reduced via phlebotomy, medications such as chelating agents, and low iron diets. Iron depletion therapy, while sucessful in reducing iron stores in iron overload due to HFE gene mutations it is less clear if this leads to a reduction is iron overload complications(Adams & Barton, 2010).
Reducing iron stores through phlebotomy is the primary method utilized in the treatment of iron overload related to HH, and has been in use for approximately 50 years(Adams & Barton, 2010). A reduction in iron stores is produced through the removal of whole red blood cells, and hemoglobin(Adams & Barton, 2010). Patients must not be at risk of anemia, and have a rate of erthyocytapheresis high enough to replace red blood cell losses between treatments(Adams & Barton, 2010). There is minimal evidence that phlebotomy therapy is effective in reducing mortality rates in homozygous C282Y carriers, but a reduction in symptoms is often reported by patients(Adams & Barton, 2010). Benefits often seen by patients undergoing phlebotomy iron depletion therapy are improvements in fatigue, blood glucose control, arthritis, and a decrease in skin hyper-pigmentation(Adams & Barton, 2010). Additonally, patients with impaired liver function often see improvements when iron stores are decreased to near physiological levels(Adams & Barton, 2010).
Medications such as chelating agents, or proton pump inhibitors(PPIs) are often used for patients who cannot undergo phlebotomy therapy. Chelating agents, such as Deferasirox, or Deferoxamine bind to iron, and then can be excreated from the body in the stool(Adams & Barton, 2010). PPIs, often prescribed for GERD or acid reflux, reduce stomach acid excretions. The increase of stomach pH effectivly reduces the asorbtion of non-heme iron(Adams & Barton, 2010). They have been shown to be effective in small clinical trials, and may partially account for the lower penetrance in C282Y homozygotes(Adams & Barton, 2010).
ii. Genetic Component
Classical Hereditary Hemochromotosis(HH) is composed of two mutations or SNPs on the HFE gene; C282Y, and H63D. Both C282Y, and H63D SNPs are autosomal recessive, meaning two copies must be present for their effects to be expressed. HFE gene mutations account for 85% of all cases of HH(Rs1800562). The HFE gene is located on chromosome 6, and is found on the short arm at location 6p21.3(Rs1800562), and is linked to the HLA-A3 locus(Rs1799945). The HFE gene regulates protein on the surface of liver and intestinal cells(HFE). HFE regulates iron absorption through the binding of tranferrin receptor 1(TR1), and through the regulation of hepcidin(HFE). Both C282Y, and H63D SNPs result in a reduction of hepcidin.
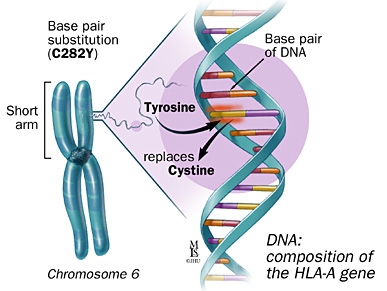
The C282Y mutation on the HFE gene is the most common, and has the largest impact(Rs1800562). The C282Y SNP consists of a point mutation from guanine to adenine resulting in a missense mutation from cysteine to tyrosine(Rs1799945). Homozygous C282Y genotypes are referenced at (A;A), while the non-carrier genotypes are referenced as (G;G)(Rs1800562).
H63D mutation, is the most common HFE mutation and is found in roughly 20% of people(Rs1799945). While more common than C282Y mutations, H63D mutations rarely result in HH, and make up only a small amount of HH cases unless found as a C282Y/H63D compound heterozygote(Rs1799945). The H63D mutation is the result of a guanine at Rs1799945 versus cystenine. A H63D homozygous genotype is referenced as (G;G), while the non carrier genotype would be referenced as(C;C)(Rs1799945).
Its believed that C282Y HFE mutations appeared 6,000 years ago in the early neolithic(Heath KM et al. 2016). The C282Y allele, is thought to have evolved due to selective pressure during climate change and an increased reliance on agriculture and a low iron environment(Heath KM et al. 2016). The increased iron absorption form the C282Y allele would have been beneficial in the high-carbohydrate rich, low iron diet found in the European neolithic(Heath KM et al. 2016). Additionally iron, plays a role in thermoregulation, and increased ferritin levels would have been useful in the cold, damp northern European environment where this mutation is prevalent(Heath KM et al. 2016).
iii. Nutrition in Hemochromotosis
The role of nutrition and iron intake is not clear in the development of HH. There currently is no randomized prospective clinical trial available on dietary interventions in HH that currently exists, but it is believed that diet plays a role(Moretti, D. et al, 2013). There is substantive differences in the absorption of heme vs non-heme iron in individuals with HH, and in comparison to non-effected individuals with both forms of iron having enhanced absorption in effected genotypes
(Moretti, D. et al, 2013). Additionally there are several several dietary compounds that may enhance or inhibit iron absorption(Adams & Barton, 2010).
Heme iron is almost exclusively found in animal products, such as beef, chicken, and eggs, and generally has much higher availability than non-heme. Heme-bound iron found in myoglobin and hemoglobin is absorbed directly by the epithelial cell in the intestinal lumen(West AR, Oates PS. 2008). This process is a form of receptor mediated endocytosis(West AR, Oates PS. 2008).
Non-heme iron is found in both animal products as well as vegetables, and has a lower level of absorption. Additionally non-heme iron is commonly included in fortified products such as breads, flours, and cereals. Absorption of non-heme iron is mediated by ferric reductase and divalent metal transporter-1 (DMT-1)(Krayenbuehl, P., T. et al. 2005). Krayenbuehl, P., T. et al. found that non-heme iron was enhanced more than heme iron in HH(2005). This was thought to be related to the up-regulated DMT-1 activity in the intestinal cells(Krayenbuehl, P., T. et al. 2005). Krayenbuehl, P., T. et al found that HH afflicted individuals had higher blood concentrations of the heavier iron isotopes, which are found in the non-heme iron types. This would indicate that non-heme iron may pose more of a risk to individuals with HH. Other studies have found that iron accumulation in HH correlates with consumption of iron-fortified foods(Adams & Barton, 2010).
Other food and non-food items that have been shown to effect iron absorption in HH include alcohol, tea, and non-citrus fruits. Alcohol, significantly decreases hepcidin expression, and results in an increase in iron abortion, as well as the additional liver damage(Adams & Barton, 2010). Black tea drank with meals has been shown to decrease the absorption of non-heme iron. This reduction in absorption is through the binding of iron with tannins(Adams & Barton, 2010). Presumably other high tannic foods would have a similar effect. Higher consumption of non-citrus fruits has been associated with upward of 20% lower serum feritin levels in HH in effected men(Adams & Barton, 2010). This association was not seen with citrus fruits. Its thought to be related to the high vitamin C levels, which enhance absorption of iron.
iv. Personalized diet for Hemochromotisis
Dietary interventions have not historically been very successful in the treatment of HH, and further studies are needed(Moretti, D. et al, 2013). Past dietary recommendations have focused on the minimization of heme-iron intake, as well as steps to limit the possibility of infections form pathogens commonly found in seafood(Adams & Barton, 2010).
A diet centered around the treatment of HH would place a limit on iron fortified foods such as breads, cereals and dishes using fortified flours as the consumption of iron fortified foods tracks well with disease progression in HH(Adams & Barton, 2010). Limits on raw shellfish maybe prudent if considerable liver damage is evident, as raw shellfish may harbor pathogens that can further damage the liver. Alcohol intake should be limited due to its ability to inhibit hepcidin and increase iron absorption further as well as damage the liver(Adams & Barton, 2010).
The consumption of non-citrus fruits, tea/coffee, as well as a verity of vegetables, both starchy and non starchy would be encouraged in individuals with HH. Tea/coffee consumed during a meal has a possibility to inhibit absorption while an increase in non-citrus fruit intake, has been shown to correlate with a lower serum ferratin level(Adams & Barton, 2010).
Although, its likely that HH iron overload may be related to the unregulated absorbtion of non-heme iron, the intake of heme iron does still play a role in disease progression. As such the consumption of high heme-iron foods such as red meat should be limited, and eaten in moderation.
Its likely that dietary interventions have a positive effect, in the treatment of HH either in addition to or as complimentary to iron depletion therapies. Clearly HH is a multivariate disease, and it is unclear why only a relatively small portion of C282Y homozygotes develop iron overload. In my opinion, diet and lifestyle, in addition to possibly unknown genetic promoters play a role in disease progression. Previous dietary interventions have focused on chelation with tea/coffee, and a reduction in heme iron, and red meat specifically due to its high bio-availability. There exists some evidence that HH iron overload may be more attenuated by non-heme iron than heme iron. With this in mind dietary interventions may find more success.
Work Cited
- Dixon, S. J., & Stockwell, B. R. (2013, December 17). The role of iron and reactive oxygen species in cell death. Retrieved from https://www.nature.com/articles/nchembio.1416
- Krayenbuehl, P., Walczyk, T., Schoenberg, R., von Blanckenburg, F., & Schulthess, G. (2005). Hereditary hemochromatosis is reflected in the iron isotope composition of blood. Blood, 105(10), 3812-3816. Accessed March 20, 2019. https://doi.org/10.1182/blood-2004-07-2807.
- Heath KM, Axton JH, McCullough JM, Harris N. The evolutionary adaptation of the C282Y mutation to culture and climate during the European Neolithic. Am J Phys Anthropol. 2016;160(1):86-101.
- West AR, Oates PS. Mechanisms of heme iron absorption: current questions and controversies. World J Gastroenterol. 2008;14(26):4101-10.
- Fleming RE, Britton RS, Waheed A, Sly WS, Bacon BR. Pathophysiology of hereditary hemochromatosis. Semin Liver Dis. 2005;25(4):411-9.
- Pilling, L. C., Tamosauskaite, J., Jones, G., Wood, A. R., Jones, L., Kuo, C. L., … Melzer, D. (2019). Common conditions associated with hereditary haemochromatosis genetic variants: cohort study in UK Biobank. BMJ (Clinical research ed.), 364, k5222. doi:10.1136/bmj.k5222
- Bacon, B. R., Powell, L. W., Adams, P. C., Kresina, T. F., & Hoofnagle, J. H. (1999). Molecular medicine and hemochromatosis: At the crossroads. Gastroenterology, 116(1), 193-207. doi:10.1016/s0016-5085(99)70244-1
- Adams, P. C., Deugnier, Y., Moirand, R., & Brissot, P. (1997, January 25). Clinical manifestations and diagnosis of hereditary hemochromatosis. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed?term=8985284
- The labile iron pool: Characterization, measurement, and participation in cellular processes. (2002, October 02). Retrieved from https://www.sciencedirect.com/science/article/pii/S0891584902010067?via=ihub
- Rs1799945. (n.d.). Retrieved from https://www.snpedia.com/index.php/Rs1799945 H63D
- Rs1800562. (n.d.). Retrieved April 22, 2019, from https://www.snpedia.com/index.php/Rs1800562
- Allen KJ et al. (2008). “Iron-overload-related disease in HFE hereditary hemochromatosis.” N Engl J Med. 358(3):221-30.
- Fleming RE, Britton RS, Waheed A, Sly WS, Bacon BR. Pathophysiology of hereditary hemochromatosis. Semin Liver Dis. 2005;25(4):411–419. doi:10.1055/s-2005-923313
- “Hemochromatosis.” Mayo Clinic, Mayo Foundation for Medical Education and Research, 5 Jan. 2018, www.mayoclinic.org/diseases-conditions/hemochromatosis/diagnosis-treatment/drc-20351448.
- Nichols, G. M., & Bacon, B. R. (1989, August). Hereditary hemochromatosis: Pathogenesis and clinical features of a common disease. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/2667334
- TIBC, UIBC, and Transferrin. (n.d.). Retrieved from https://labtestsonline.org/tests/transferrin-and-iron-binding-capacity-tibc-uibc
- Bryant, J., Cooper, K., Picot, J., Clegg, A., Roderick, P., Rosenberg, W., & Patch, C. (2009). Diagnostic strategies using DNA testing for hereditary haemochromatosis in at-risk populations: A systematic review and economic evaluation. Health Technology Assessment, 13(23). doi:10.3310/hta13230
- Adams, P. C., & Barton, J. C. (2010). How I treat hemochromatosis. Blood, 116(3), 317-325. doi:10.1182/blood-2010-01-261875
- HFE gene – Genetics Home Reference – NIH. (n.d.). Retrieved April 22, 2019, from https://ghr.nlm.nih.gov/gene/HFE
- Moretti, D., Doorn, G. M., Swinkels, D. W., & Melse-Boonstra, A. (2013). Relevance of dietary iron intake and bioavailability in the management of HFE hemochromatosis: A systematic review. The American Journal of Clinical Nutrition, 98(2), 468-479. doi:10.3945/ajcn.112.048264